From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. This means the electrons are easier to remove because the nucleus does not hold them as strongly.

The Parts Of The Periodic Table
120 rows First Second and Third Ionization Energy eV First Ionization Energy.
. Which metal has lowest ionization energy. It becomes harder to remove an electron as the atomic radius decreases because the electrons are generally closer to the nucleus which is also more positively charged. Calculate the ionization energy in units of electron volts for a one-electron atom by squaring Z and then multiplying that result by 136.
The elements that belong to the noble gases or inert gases or Group VIII-A have the highest ionisation energy. Group I elements have low ionization energies because the loss of an electron forms a stable octet. What is the first ionization energy.
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. Ionization energy of Helium He 2458 eV. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group.
Answer and Explanation. Determine the location of the electron. How to Determine the Valence Orbital of an Element.
Cesium has the smallest ionization energy since it has the largest amount of energy levels creating a smaller attractive force making it easier to remove the electron. Based on that information the correct arrangement would be In Sn Te Xe. How to Calculate the Ionization Energy of Atoms Determine what atom you want to use for calculating the ionization energy.
The element with the lowest ionization energy is cesium Cs. 119 rows The chemical elements of the periodic chart sorted by. Decide how many electrons the atom contains.
By the difference between the photon energy and ejected electron energy ionization energy is calculated. It takes very little energy to remove an electron because the atom can be more stable without it. There is more shielding between the nucleus and the outer electrons and the distance between the nucleus and the outer electron increases and therefore the force of attraction between the nucleus and outer most electrons is reduced.
Which elements have the smallest ionization energy. No matter the charge of a nucleus if the electron is far enough away its attraction is much weaker. Electrons are ejected from atoms and their kinetic energy are measured.
Calculate the ionization energy in units of electron volts for a one-electron atom by squaring Z and then multiplying that result by 136. The lattice energy of NaCl for example is 788 kJmol while that of MgCl2 is 1080 kJmol. If you must determine which element from a list has the highest ionization energy find the elements placements on the periodic table.
Find the distance between the electron and the nucleus. Cesium has atomic number 55 and is in the fifth row of the periodic table. How do you find ionization energy.
55 - Atomic number. Decide how many electrons the atom contains. Thus the first ionization energy is smaller.
The ionization energy may be an indicator of the reactivity of an element. The greater the ionization energy the more difficult it is to remove an electron. The general trend of ionization energy is increasing from left to right and bottom to top following the atomic radius trend which means that the bottom left elements would have the lowest ionization energy.
This graph shows the first ionization. Ionization energy of Lithium Li 539 eV. Determine what atom you want to use for calculating the ionization energy.
The first or initial ionization energy or E i of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated. Why does Group 1 have the lowest ionization energy. Hence this will have the highest ionization energy.
Ionization energy of Beryllium Be 932 eV. Going down the group the first ionisation energy decreases. 5441 eV 3.
As the attraction of the nucleus decreases with increasing orbit number Ar 3d 10 4s 2 4p 2 will have the lowest ionization energy. Ionization energy of Hydrogen H 1359 eV 2. On the opposite end of the spectrum group 17 elements have very high ionization energies.
Therefore a greater first ionization energy is needed as the nucleus becomes more positively charged. Remember that elements near the top of the periodic table and further to the right of the periodic table have higher ionization energies. Among the rest Ne 3s 2 3p 3 is more stable due to half-filled orbitals.
If we were to take a single element then Helium is said to have the highest first ionization energy among all the other neutral elements. Thus group 1 elements have very low ionization energies. Thus helium has the largest first ionization energy while francium has one of the lowest.
Another way is by irradiating gaseous atoms with UV or X-Ray monochromatic radiation frequency must be chosen to provide photons with energy above ionization energy. If you follow the general trend on the periodic table you see that ionization energy decreases down a period because as electrons are added to higher octets the average distance of the electron from the nucleus increases and screening by inner electrons increases. This is because with 7 valence electrons halogens want to gain one more electron to form an octet.
Elements with a low ionization energy tend to be reducing agents and form cations which in turn combine with anions to form salts. 37 - Atomic Mass. This is due to the fact that the later bonds have a.
Atomic number - Name alphabetically.
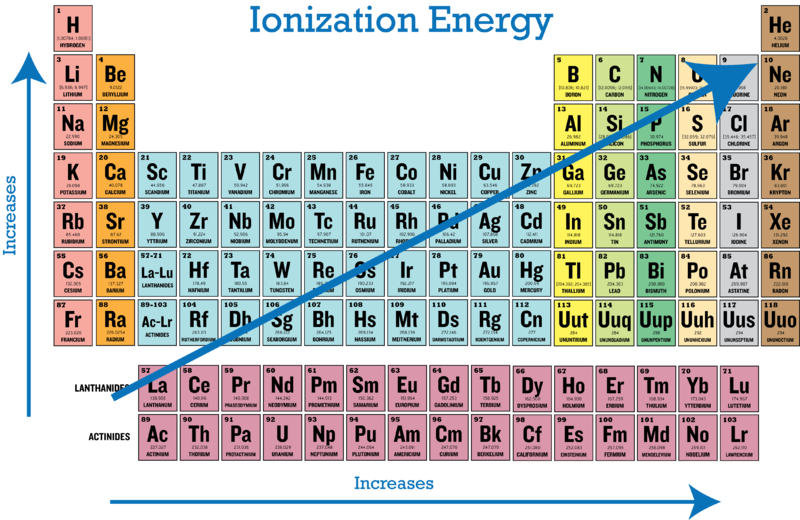
Periodic Trends In Ionization Energy Ck 12 Foundation
0 Comments